EDCTP Fellows in the times of COVID-19
Our EDCTP Fellows have been very active across the continent in the response to the COVID-19 pandemic. Many countries around the world have been in varying degrees of lockdown and have relied heavily on advice from medical specialists, epidemiologists and researchers to guide their response. Please read our EDCTP monthly updates as we showcase the work of our Fellows each month https://www.edctp.org/web/app/uploads/2020/08/EDCTP-Update-July-2020.pdf
Bourema Kouriba - COVID-19 diagnosis in Mali
Professor Bourema Kouriba (a former EDCTP1 senior Fellow) is a pharmacist, immunologist, and the Director General of the Charles Mérieux Centre for Infectiology (CMCI) in Bamako, Mali. He leads a team responsible for diagnostics of emerging and re-emerging infectious diseases and teaches immunology at the Faculties of Medicine and Pharmacy in Bamako.
Since the Ebola epidemic in 2014, the institute developed its diagnostics capabilities with the support of the Institute of Microbiology of the German Army. The German government donated a mobile laboratory to the Malian Ministry of Health under the responsibility of CMCI, which participates with the National Institute of Public Health in epidemiological surveillance and the response to epidemics. Professor Kouriba: "On the outbreak of the COVID-19 pandemic in March 2020, CMCI and three other
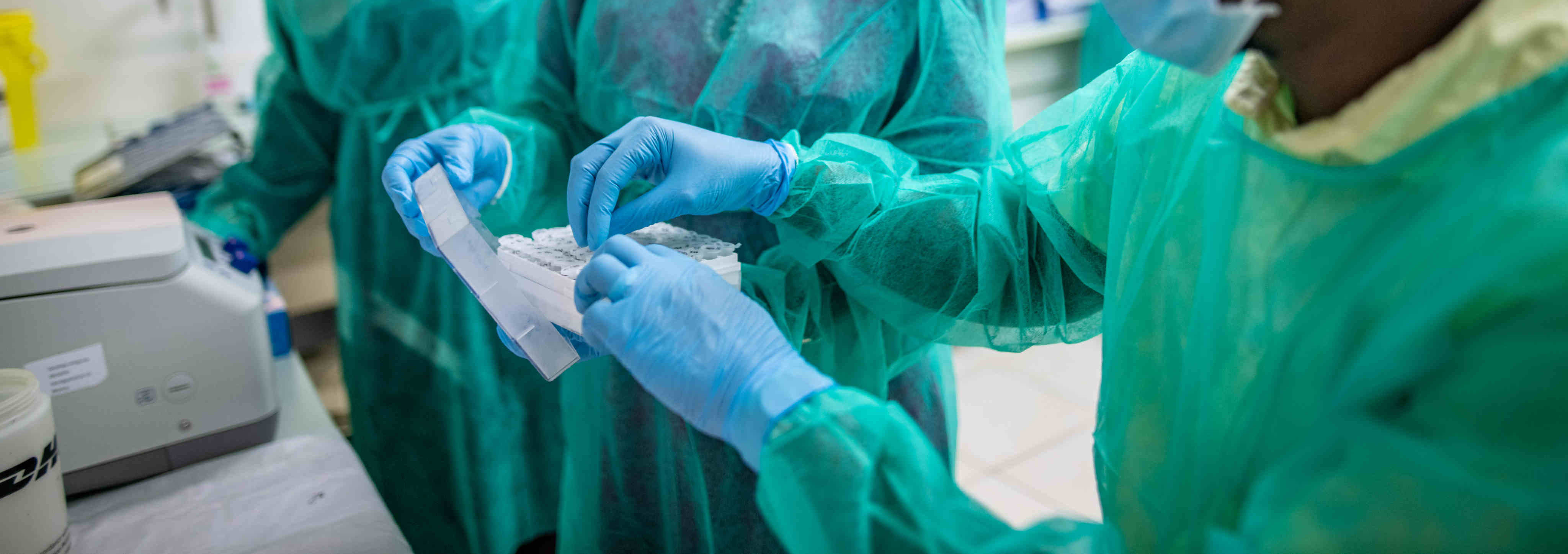
centres were appointed by the Ministry of Health and Social Affairs to carry out the diagnostic side. Right now, we are in Timbuktu, a town in the north at 900 km from the capital Bamako. Here, our mobile laboratory is deployed to carry out the COVID19 molecular diagnostic tests. The day begins with the preparation of bleach solutions. Then we organise the team in the different workstations: one person at the reception, two people in the glove box, one person at the RNA extraction and the preparation of the master mix and another at the amplification."
"I take care of entering data and editing the results personally. As soon as the samples are received, their number is counted and communicated to the entire team. Once received, samples are conditioned in a container and transferred to the glove box team whose role is to inactivate these samples. This inactivation is carried out under the control of a witness who notes on the patient form all the steps performed by the operator. After inactivation, the samples are removed from the glove box for RNA extraction. At the same time, one person responsible for preparing the master mix prepares the necessary quantity of reagent. The last step is the real-time PCR. After two hours the results are analysed, interpreted, entered into the
database and printed for the doctor. At the end of the day, the glove compartment and the equipment are cleaned with concentrated bleach." There are several serious challenges:
1. The situation of consumables used for PCR, in particular for extraction kits. With the borders closed, the supply of reagents and consumables in the country is a very big challenge.
2. We receive most of our reagents through donors as the government cannot afford to buy them. Each donor buys reagents, which poses a problem for having a uniform algorithm to interpret the results.
3. Non-compliance with distancing and prevention measures which increases the number of contact cases, hence the number of samples to be tested per day. The laboratory works at least 10 hours a day to meet demand.
"We are in an unprecedented pandemic against which we are actually learning and responding at the same time. Timbuktu is in the north in an insecure area of Mali and the temperature fluctuates between 42 and 46 degrees Celsius. But it is our population and we must guarantee equity in access to care. Everyone should take this pandemic seriously and show solidarity with countries with limited resources because SARS-COV2 does not know borders and does not differentiate between people. Countries must be united in the fight against COVID-19."
Articles
Test Article With Image
Test article 14 July 2025 with image TL
Read moreVirtual Workshop: Recognition And Control Of Mtb Infected Cells: From Basics To The Clinic | 13 – 14June 2023
The Stop
TB Partnership Working Group on New TB Vaccines (WGNV) and the National
Institute for Allergy and Infectious Diseases (NIAID)are
co-hosting a workshop on the topic of Recognition and Control of Mtb
Infected Cells: From Basics to the Clinic. This workshop is intended to address
the need for correlates and to identify platforms that measure recognition or
control of the infected cell - especially in humans - as identified in the EDCTP/AIGHD Global Roadmap for
Research and Development of New TB Vaccines and the Strategic Framework for
New TB Vaccines in the Stop TB Partnership Global Plan to End TB 2023 - 2030.
The overall goals of this workshop
are to:
- elucidate the mechanisms by
which the immune system recognizes the Mtb-infected cell
- explore the degree to which
such recognition can lead to control of the intracellular microbe, and
- discuss the translational
implications of these observations
The workshop format will be largely
discussion-based. Each session will have three short presentations that will
provide a high-level overview of key topics within the session theme, followed
by a discussion between the session chairs, speakers, and participants.
Click here
to view the programme and
speakers.
This workshop is free of charge and
open to any participants interested in this topic.
Ssi/Isid Infectious Diseases Research Fellowships | Deadline For Applications: 16 June 2023
The International Society for Infectious Diseases (ISID) and the Swiss
Society for Infectious Diseases (SSI) are inviting applications for their joint
Infectious Diseases Research Fellowship Program. The purpose of this programme
is to support infectious disease physicians and scientists from under-resourced
countries through multidisciplinary clinical and laboratory training at a
select biomedical institution in Zurich, Switzerland. The one-year SSI/ISID
Fellowship programme is open to applicants who are 40 years or younger,
citizens and permanent residents of under-resourced countries or Eastern
Europe. The deadline for applications is 16 June 2023.
More information: https://na.eventscloud.com/eSites/748378/Homepage