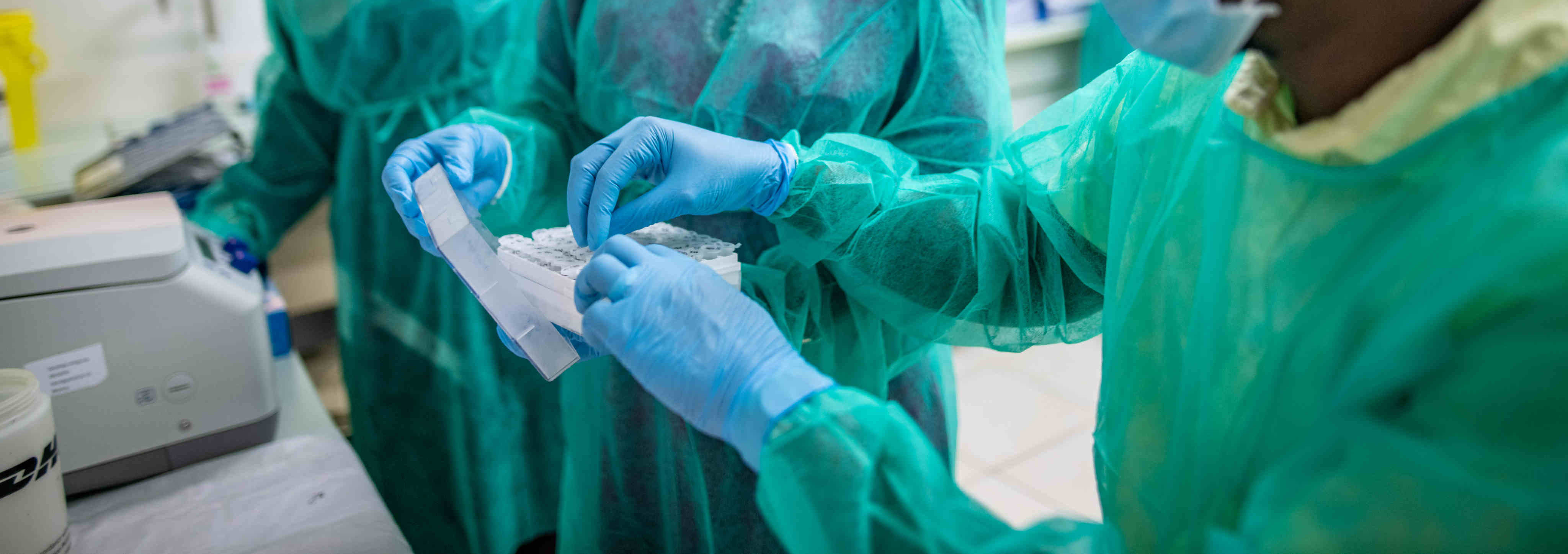
EDCTP celebrates World Malaria Day!
On World Malaria Day 2021, EDCTP joins all partners in the ‘Zero Malaria – Draw the Line Against Malaria’ campaign towards the goal of malaria elimination. Towards malaria research & development, EDCTP has invested more than €150 million to support a portfolio of 64 research projects conducted by African-European consortia and research fellows in Africa.
These projects support the development of innovative products – diagnostics, drugs and vaccines – needed to manage, control and eliminate malaria. EDCTP funding progressed the development of the R21 vaccine that recently demonstrated greater than 75% protection in a recent phase II trial. The EDCTP malaria portfolio also includes studies on implementation of interventions and activities to strengthen health systems, essential to ensure successful roll-out of new and existing products, and to contribute to the malaria eradication agenda.
“We must not waver in our efforts to control and steadily move towards malaria eradication, despite the COVID-19 pandemic and other challenges. The recent exciting results from the clinical development of the R21 malaria vaccine in Burkina Faso bring us a step closer to a highly effective malaria vaccine. We remain committed to support the development of new and improved products for the diagnosis, prevention and treatment, as well as product-focused implementation research to ensure that these products reach the populations that need them most.”
Dr Michael Makanga, EDCTP Executive Director
For more about EDCTP funded malaria projects visit https://www.edctp.org/news/world-malaria-day-2021/
Articles
Test Article With Image
Test article 14 July 2025 with image TL
Read moreVirtual Workshop: Recognition And Control Of Mtb Infected Cells: From Basics To The Clinic | 13 – 14June 2023
The Stop
TB Partnership Working Group on New TB Vaccines (WGNV) and the National
Institute for Allergy and Infectious Diseases (NIAID)are
co-hosting a workshop on the topic of Recognition and Control of Mtb
Infected Cells: From Basics to the Clinic. This workshop is intended to address
the need for correlates and to identify platforms that measure recognition or
control of the infected cell - especially in humans - as identified in the EDCTP/AIGHD Global Roadmap for
Research and Development of New TB Vaccines and the Strategic Framework for
New TB Vaccines in the Stop TB Partnership Global Plan to End TB 2023 - 2030.
The overall goals of this workshop
are to:
- elucidate the mechanisms by
which the immune system recognizes the Mtb-infected cell
- explore the degree to which
such recognition can lead to control of the intracellular microbe, and
- discuss the translational
implications of these observations
The workshop format will be largely
discussion-based. Each session will have three short presentations that will
provide a high-level overview of key topics within the session theme, followed
by a discussion between the session chairs, speakers, and participants.
Click here
to view the programme and
speakers.
This workshop is free of charge and
open to any participants interested in this topic.
Ssi/Isid Infectious Diseases Research Fellowships | Deadline For Applications: 16 June 2023
The International Society for Infectious Diseases (ISID) and the Swiss
Society for Infectious Diseases (SSI) are inviting applications for their joint
Infectious Diseases Research Fellowship Program. The purpose of this programme
is to support infectious disease physicians and scientists from under-resourced
countries through multidisciplinary clinical and laboratory training at a
select biomedical institution in Zurich, Switzerland. The one-year SSI/ISID
Fellowship programme is open to applicants who are 40 years or younger,
citizens and permanent residents of under-resourced countries or Eastern
Europe. The deadline for applications is 16 June 2023.
More information: https://na.eventscloud.com/eSites/748378/Homepage